Manual Massage Foot Spa Massager A manual massage Foot Spa Massager is a device designed to provide a relaxing and therapeutic foot massage. It is a manually operated handheld device, which means it does not require electricity or batteries to operate. The massager is made up of several parts, including the handle, rollers, and knobs. Manual Massage Foot Spa Massager,Portable Pedicure Tub,Bath Foot Massager,Foot Bath Machine Huaian Mimir Electric Appliance Co., LTD , https://www.footspamachine.com













To use the manual massage foot spa massager, you simply place your foot on a flat surface and apply the massager to the soles of your feet. The rollers and nodules stimulate the nerves and muscles in the foot, providing a deep tissue massage that helps relieve tension and pain.
The massager is small and lightweight, so you can easily take it with you wherever you go. It's also easy to clean, just wipe with a damp cloth after each use.
Overall, the Manual Massage Foot Spa Massager is a great option for those who are looking for an easy and effective way to relax and soothe their feet. It's affordable, easy to use, and can provide a range of health benefits, including improving circulation, reducing stress, and relieving foot pain and discomfort.
Determination of Tigecycline in Plasma by High Performance Liquid Chromatography Tandem Mass Spectrometry
Determination of Tigecycline in Plasma by High Performance Liquid Chromatography Tandem Mass Spectrometry
Zhou Liang
Thermo Fisher Scientific (China) Co., Ltd.
1. Introduction
Tigecycline, also known as 9-tert-butylglycinamide minocycline, butanocycline, is the first glycyl-tetracycline antibiotic approved for clinical intravenous administration. It has an ultra-broad spectrum of antibacterial activity, including Gram-positive and Gram-negative aerobic bacteria and anaerobic bacteria and other important pathogenic bacteria. The mechanism of action of tigecycline is similar to that of tetracycline antibiotics. It binds to the 30S ribosome of bacteria and prevents the entry of transfer RNA, making it impossible for amino acids to bind to peptide chains, ultimately blocking bacterial protein synthesis and limiting bacterial growth. effect. Tigecycline can overcome or limit the role of bacterial efflux pump and ribosome protection, and is not easy to produce drug resistance. Its broad tissue distribution and long-term elimination half-life pharmacokinetic properties are optimistic. At the same time, it is not necessary to adjust the dose and time for patients with renal dysfunction and hemodialysis. In June 2005, the US FDA approved the use of adult complex skin and soft tissue infections and complex intra-abdominal infections in adults, but it is still in clinical research and declared production stage in China.
In this experiment, an LCMSMS method for the determination of tigecycline in plasma was established. The appropriate sample pretreatment method was used. The impurities in the plasma did not interfere with the determination of the sample, and the sensitivity was high and the reproducibility was good.
2 . Experimental part
2.1 Instruments and reagents:
TSQ Vantage Triple Quadrupole Mass Spectrometer (Thermo Fisher Scientific), equipped with electrospray ionization source (ESI); Dionex Ultimate 3000 RSLC ultra high pressure liquid chromatography.
Standards: The purity of tigecycline and internal standard tetracycline hydrochloride were both greater than 98%. See Table 1 for information. Dissolve in methanol and prepare a standard stock solution of 1.0 mg/mL, and then dilute to a suitable concentration of mixed standard working solution as needed. All other reagents were HPLC chromatographically pure.
Table 1. Compound information to be tested
2.2 Instrument method:
2.2.1 Liquid phase conditions:
Column: Hypersil GOLD C18, 100mm x 2.1mm;
The gradient elution procedure is shown in Table 2, and the injection volume is 5 μL.
Table 2. LC Gradient Conditions
Time /min
Water (0.2% formic acid + 5 mM ammonium formate )
Acetonitrile
Flow rate / μL/min
0.00
95
5
400
3.00
20
80
400
3.50
20
80
400
3.60
95
5
400
5.00
95
5
400
2.2.2 Mass spectrometry conditions:
Electrospray ionization source (ESI), positive ion mode; selective reaction monitoring (SRM) scanning mode, spray voltage: 3000V; gasifier temperature 320 ° C, ion transfer tube temperature: 350 ° C; nitrogen as sheath gas and auxiliary gas, of which The sheath gas is 35 arb, the auxiliary gas is 10 arb, the argon gas is used as the collision gas, and the collision pressure is 1.5 mTorr; the reaction monitoring (SRM) is selected, and the ion pair information is shown in Table 3.
Table 3. Ion pair information in SRM mode
Among them, tigecycline was selected as m/z 513.2, and tetracycline was selected as m/z 410.2 as a quantitative ion.
2.3 plasma sample processing method:
Add 10 μL of internal standard solution (100 ng/mL tetracycline solution) to 0.2 mL of plasma, add 100 μL of 20% trichloroacetic acid (TCA) to precipitate protein, vortex for 1 min, centrifuge for 10 min (13000 r/min), and take 5 μL of supernatant for injection. analysis.
3. Methodology verification results analysis
3.1 Specificity:
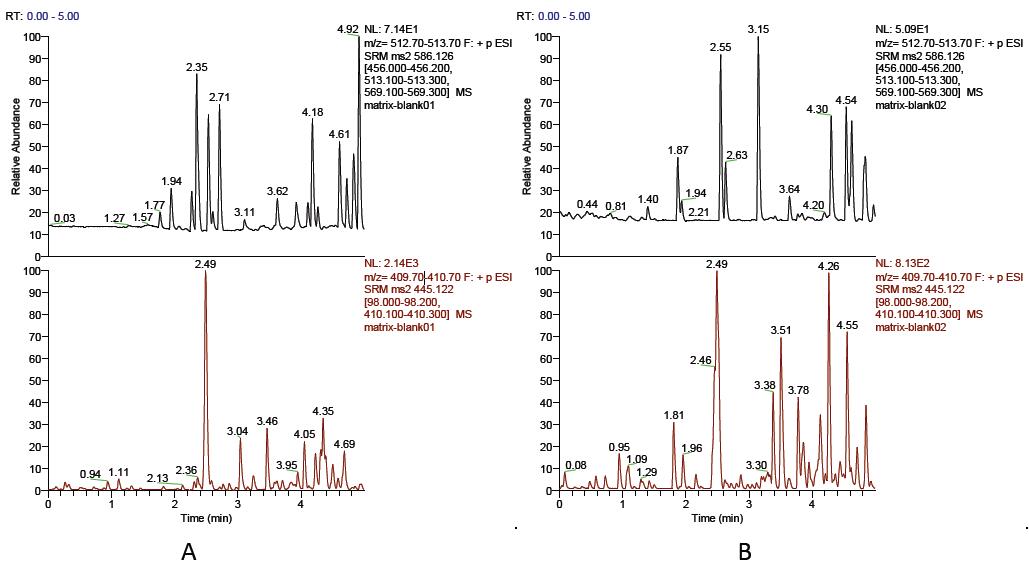
Under the test conditions used in this experiment, tigecycline peaked around 1.98min, the internal standard tetracycline peaked around 2.49min, the peak shape was good, no impurity peak interference was measured, and the baseline was stable (see Figure 1). The method has high specificity and can accurately determine the concentration of tigecycline in plasma with high sensitivity.
Figure 1. Chromatogram of tigecycline and internal standard tetracycline in plasma
(AF): Chromatogram of blank plasma from 6 different sources;
(G): Chromatogram of blank plasma added to known concentrations of tigecycline and tetracycline
3.2 Minimum Detection Limit (LLOD)
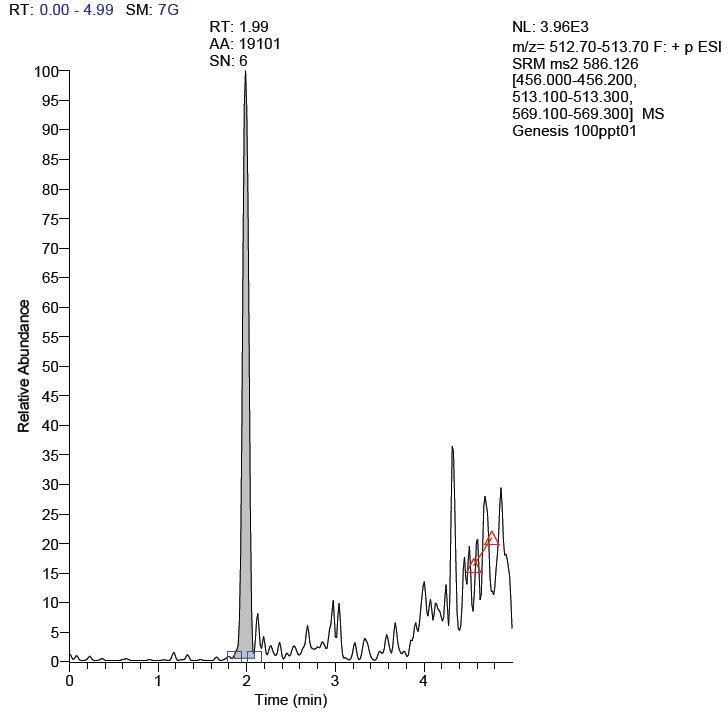
10 μL of tigecycline standard solution was added to the blank plasma to prepare 0.2 mL of standard drug-containing plasma at a concentration of 100 ppt, and the chromatogram was recorded under the "plasma sample treatment" to obtain the tigecycline plasma matrix. The minimum detection limit for the spike, S/N=6, and the result is shown in Figure 2.
Figure 2. Chromatogram of tigecycline LLOD
3.3 standard curve and quantitative range
The 1 mg/mL tigecycline solution was diluted stepwise into a series of concentrations, and 10 μL of each was added to the blank plasma to prepare 0.2 mL of standard drug-containing plasma at concentrations of 5, 10, 50, 100, 500, 1000, and 2000 ppb, respectively. Under the operation of plasma sample processing, the peak area of ​​each standard point and internal standard is recorded. The concentration is the abscissa, the ratio of the peak area of ​​the analyte to the internal standard is the ordinate, and the regression calculation is performed by the weighted least squares method to obtain the linear regression equation. It is a standard curve and is operated in parallel three times. The results are shown in Table 4 and Figure 3.
Table 4. Tigecycline standard curve (n=3)
Figure 3. Tigecycline standard curve
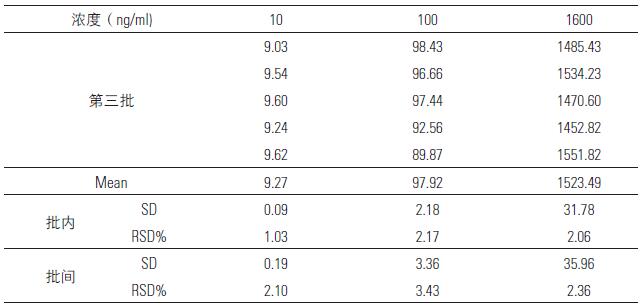
3.4 precision and accuracy
Different amounts of tigecycline were added to the blank plasma to prepare 0.2 mL of standard drug-containing plasma at concentrations of 10, 100, and 1600 ppb, respectively, and operated under "plasma sample processing", within the batch and between batches. Five samples were prepared for each concentration, the chromatogram was recorded, the concentration was calculated, and the intra- and inter-assay precision was obtained. The results are shown in Table 5.
Table 5. Intra-day and inter-day precision of tigecycline in plasma (n=5)
Different amounts of tigecycline were added to the blank plasma to prepare 0.2 mL of standard drug-containing plasma at concentrations of 10, 100, and 1600 ppb, respectively, and operated under "plasma sample processing" during the day and day. Five samples were prepared for each concentration, the chromatogram was recorded, the concentration was calculated, and compared with the standard concentration added. The results are shown in Table 6.
Table 6. Accuracy of tigecycline in plasma (n=5)
3.5 stability
Different amounts of tigecycline were added to the blank plasma to prepare 0.2 mL of standard drug-containing plasma at concentrations of 10, 100, and 1600 ppb, respectively, and operated under "plasma sample processing" for each concentration. 25 samples, 5 parts were placed in the autosampler for 24h; 5 parts were placed at room temperature for 4h, then treated under “plasma sample treatment†for injection analysis; 5 parts were taken after -20°C freezing after preparation. Naturally thawed, then frozen and then thawed (repeated freeze-thaw cycles) and then sampled under “plasma sample processingâ€; 5 samples were frozen at -80 °C for two weeks and then operated under “plasma sample treatmentâ€. Sample analysis; 5 parts were frozen at -80 °C for four weeks and then processed under the "plasma sample treatment", injection analysis, the results are shown in Table 7.
Table 7. Stability of tigecycline in plasma (n=5)
3.6 recovery rate
Different amounts of tigecycline were added to the blank plasma to prepare 0.2 mL of standard drug-containing plasma with a concentration of 10, 100, and 1600 ppb respectively. Operate under the "plasma sample treatment" to record the peak area and inside of the sample. The peak area Æ’x(Æ’x=As'/Ai); 10,100,1600ppb sample directly recorded the ratio of peak area to internal standard peak area Æ’s(Æ’s=As/Ai), and the ratio of Æ’x to Æ’s is calculated. The recovery rate of the cyclocycline was operated 5 times in parallel, and the results are shown in Table 8.
Table 8. Recovery of tigecycline in plasma (n=5)
4. Methodology summary
In this experiment, an LC-MS/MS method for the determination of tigecycline in plasma was established. The appropriate sample pretreatment method was used. The impurities in the plasma did not interfere with the determination of the sample. The linear range of the standard curve was 5 ppb-2000 ppb, and the linear relationship was good. The minimum limit of quantification is 5 ppb; the inter-assay and intra-assay variability of the high, medium and low concentrations confirmed by the methodology are less than 10%; the accuracy is 95.05%-102.35%; the recovery rate is 93.96%-105.63%; plasma The tigecycline was placed at room temperature for 4 h, repeatedly frozen and thawed three times, and the treated sample was placed in an autosampler for 24 h and frozen in a -80 °C refrigerator for two weeks and four weeks. The sample was still stable and met the biological sample determination requirements.
5. Plasma sample determination results
The concentration of tigecycline in the plasma is shown in Table 9 after the subject has given the drug tigecycline several times in succession.
Table 9. Subject's tigecycline plasma drug concentration
6. Conclusion:
The TSQ Vantage Triple Quadrupole LC/MS system has the advantages of high sensitivity and specificity in the detection of tigecycline in plasma. The detection limit can be 100ppb; the method has a wide linear range (5ppb-2000ppb), good Precision and accuracy; simultaneous treatment of plasma samples with 20% trichloroacetic acid precipitation protein, simple and fast, blank matrix does not interfere, the recovery rate can reach 93.96%-105.63%, which proves that the method detects plasma tigeine It has good applicability.