Clear Silicone Cord,Power Cord Clips,Replacement Power Cords Xiqi Xinhuan Technology Co., Ltd. , http://www.sz-tattoo.com
Dimenhydrinate injection 50mg5mlÂ
Precautions
1. Cross-allergies Allergies to other ethanolamines may also be allergic to the drug.
2. Contraindications (1) Allergic to this drug and other antihistamines. (2) Glaucoma patients. (3) Chronic lung disease patients (such as chronic bronchitis, emphysema, etc.) (foreign data). (4) dysuria due to benign prostatic hyperplasia. (5) Newborns and premature infants are disabled.
3. Use asthma patients with caution (foreign data).
4. The impact of drugs on children Children under the age of 12 should be cautious in their use of drugs. Newborns and preterm infants should not use this drug.
5. The influence of drugs on the elderly The incidence of adverse reactions (drowthiness, dizziness, etc.) after the elderly medication is higher than that of adults, so it should be used with caution.
6. The effect of drugs on pregnancy Pregnant women take this medicine within 4 months of the first trimester of pregnancy. The incidence of congenital cardiovascular abnormalities and groin hernias in the fetus may increase, and the relationship with the medication has yet to be confirmed. Therefore, pregnant women should not use the drug. According to foreign data, a large number of pregnant women and women giving birth had not observed any data to prove that the frequency of malformations increased, or had a direct (or indirect) effect on the fetus.
7. The effect of drugs on breastfeeding Breast-feeding women should use this drug with caution.
8. The drug on the test value or diagnosis of the effect of the drug can interfere with the determination of theophylline, causing false increase in theophylline.
Adverse reactions
1. Common expressions of dullness, unresponsiveness, sleepiness, lack of concentration, fatigue, dizziness, nausea, vomiting, stomach upset, loss of appetite.
2. Uncommon hallucinations, decreased night vision, decreased physical vision, dysuria, rash, or extrapyramidal symptoms.
 [Foreign adverse reaction reference] 1. Common sedation of the central nervous system. The use of the recommended dose has a tendency to induce psychotic reactions, rare illusions and slang.
 2. Skin There have been reports of mixed drug eruptions.
 3. Eyes It has been reported that after application, color discrimination can be affected, and the visual reaction time and stereoscopic images at night can be degraded.
4. Others The long-term abuse of the drug can lead to psychological dependence and possible physiological dependence.
Drug interactions · Drug-drug interactions
1. The drug can enhance the effects of ethanol, central nervous system inhibitors (such as sedative hypnotics), and tricyclic antidepressants, and should be avoided.
 2. The drug can temporarily affect the absorption of barbiturates and sulfacetamide sodium.
 3. In combination with paraamino salicylate, the plasma concentration of the latter can be reduced. Drug-alcohol/nicotine interactions Drink alcohol-containing beverages while taking medications can increase adverse effects such as sedation, so alcohol is prohibited during medication.
 Administration
1. This drug is incompatible with the following drugs: aminophylline, opioid alkaloids, ammonium chloride, butorphanol, glycopyrronium bromide, hydrocortisone sodium succinate, prednisolone , meglumine, midazolam, pentobarbital, pentobarbital, phenobarbital, phenytoin, hydroxyzine, prochlorperazine, promazine, promethazine, chlorpromazine, trifluoro L-azine, streptomycin, tetracycline, novobiocin sodium, thiopental sodium, heparin sodium.
 2. When taking this medicine while eating or drinking milk, it can reduce the stimulation of the stomach by the medicine.
 The antiemetic effects of this drug can cause difficulties in the diagnosis of certain diseases, such as appendicitis and certain drug-induced reactions.
 4. It is forbidden to engage in high-altitude operations such as driving cars, boats, and operating machinery and equipment during drug use.
5. Overdose performance: Vomiting, dizziness, convulsions, purpura, coma, or even respiratory failure. When the amount of poisoning is reached, severe acaridosis similar to atropine poisoning may occur, accompanied by extrapyramidal symptoms, clozarizine may be administered, and symptomatic treatment such as infusion may be performed. [Usage and dosage]
The recommended dose for intramuscular injection in adults is 50 mg once every 4-6 hours. It is also possible to give 100 mg every 4 hours (no effect if this dose is associated with lethargy). ·Intravenous injection Before use, 1ml (50mg) of this drug must be diluted with 0.9% sodium chloride for injection 10ml, and the administration time is more than 2 minutes. In patients with renal insufficiency who do not have renal failure, evidence of dose reduction is needed. Hepatic insufficiency at doses of acute liver dysfunction should consider reducing the dose.
Children · Intramuscular injection The recommended dose is once 1.25mg/kg, 4 times a day (to 300mg a day). Intravenous Injection It has been reported that before surgery, the drug (50 mg/ml) is diluted to 10 ml with normal saline, and 0.5 mg/kg is injected immediately after induction of anesthesia, which can effectively reduce postoperative nausea and vomiting. In patients with renal insufficiency who do not have renal failure, evidence of dose reduction is needed. Hepatic insufficiency at doses of acute liver dysfunction should consider reducing the dose.
Storage
Protected from light, closed.
Product photo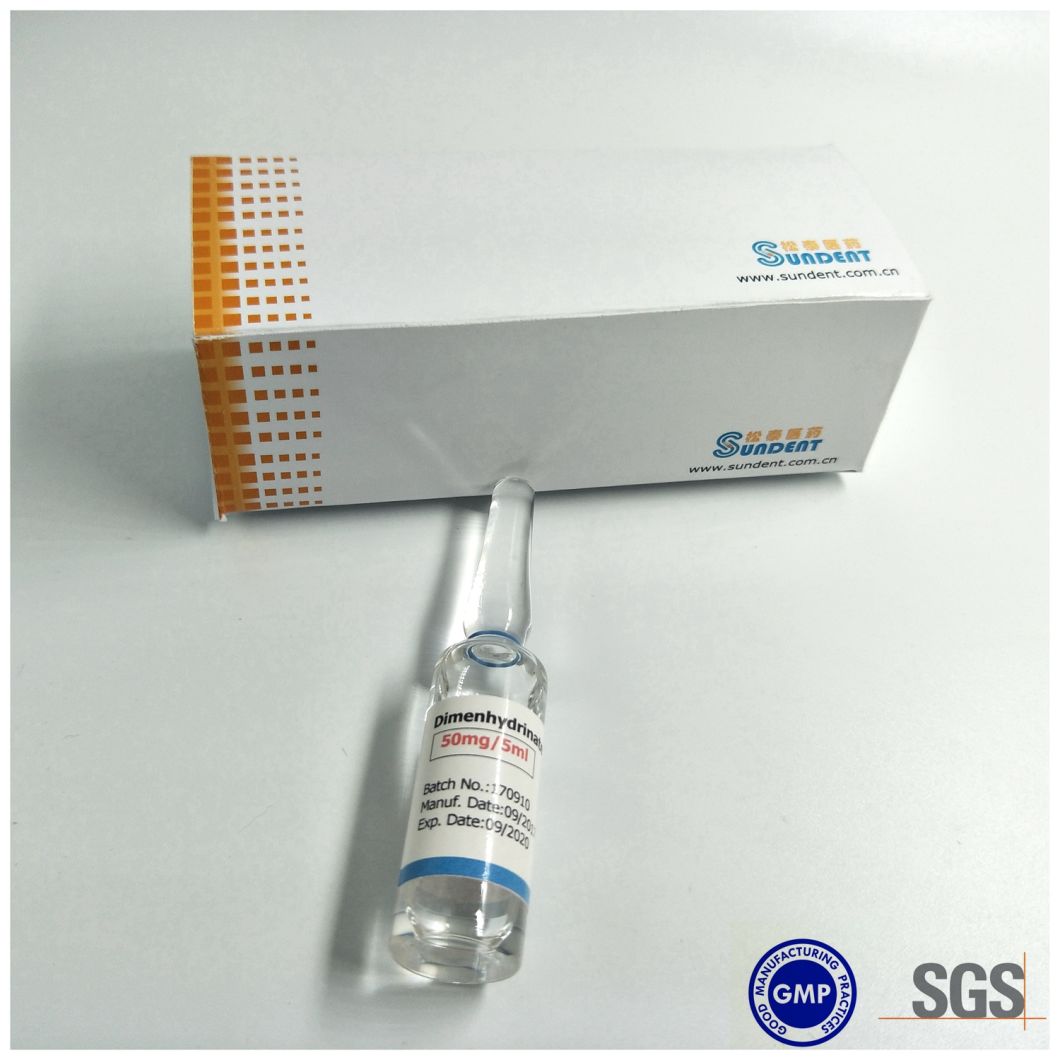
Our factory
This product is produced in our GMP factory, all our facility has past China GMP, and also officially certificated by more than ten countries'Â MOH. 
Our Service
We can specially develop the products per customers' specification.
We can offer OEM service with customers' private brands and labels.
We can offer excellent after-sale service and professional technical support.
Our Quality control system
In manufacturing, quality is what sustains our life. Starting from the material selection, there are many factors that contribute to the quality control process.  Our manufacturing facility is GMP certified. Quality Control (QC) and monitoring procedures are built into every aspect of our work.
Our Quality Control System is outlined below:
Our Registration Team and technical support
We have experienced team to provide qualified and professional registration documents for most of countries. Up to present, we have registered more than hundred products and exported to more than 20 countries. Especially including those in Asia, Africa, South America, Middle east area and East Europe.
We can provide regulatory documents
Description
Dimenhydrinate injection 50mg5mlÂ
Precautions
1. Cross-allergies Allergies to other ethanolamines may also be allergic to the drug.
2. Contraindications (1) Allergic to this drug and other antihistamines. (2) Glaucoma patients. (3) Chronic lung disease patients (such as chronic bronchitis, emphysema, etc.) (foreign data). (4) dysuria due to benign prostatic hyperplasia. (5) Newborns and premature infants are disabled.
3. Use asthma patients with caution (foreign data).
4. The impact of drugs on children Children under the age of 12 should be cautious in their use of drugs. Newborns and preterm infants should not use this drug.
5. The influence of drugs on the elderly The incidence of adverse reactions (drowthiness, dizziness, etc.) after the elderly medication is higher than that of adults, so it should be used with caution.
6. The effect of drugs on pregnancy Pregnant women take this medicine within 4 months of the first trimester of pregnancy. The incidence of congenital cardiovascular abnormalities and groin hernias in the fetus may increase, and the relationship with the medication has yet to be confirmed. Therefore, pregnant women should not use the drug. According to foreign data, a large number of pregnant women and women giving birth had not observed any data to prove that the frequency of malformations increased, or had a direct (or indirect) effect on the fetus.
7. The effect of drugs on breastfeeding Breast-feeding women should use this drug with caution.
8. The drug on the test value or diagnosis of the effect of the drug can interfere with the determination of theophylline, causing false increase in theophylline.
Adverse reactions
1. Common expressions of dullness, unresponsiveness, sleepiness, lack of concentration, fatigue, dizziness, nausea, vomiting, stomach upset, loss of appetite.
2. Uncommon hallucinations, decreased night vision, decreased physical vision, dysuria, rash, or extrapyramidal symptoms.
 [Foreign adverse reaction reference] 1. Common sedation of the central nervous system. The use of the recommended dose has a tendency to induce psychotic reactions, rare illusions and slang.
 2. Skin There have been reports of mixed drug eruptions.
 3. Eyes It has been reported that after application, color discrimination can be affected, and the visual reaction time and stereoscopic images at night can be degraded.
4. Others The long-term abuse of the drug can lead to psychological dependence and possible physiological dependence.
Drug interactions · Drug-drug interactions
1. The drug can enhance the effects of ethanol, central nervous system inhibitors (such as sedative hypnotics), and tricyclic antidepressants, and should be avoided.
 2. The drug can temporarily affect the absorption of barbiturates and sulfacetamide sodium.
 3. In combination with paraamino salicylate, the plasma concentration of the latter can be reduced. Drug-alcohol/nicotine interactions Drink alcohol-containing beverages while taking medications can increase adverse effects such as sedation, so alcohol is prohibited during medication.
 Administration
1. This drug is incompatible with the following drugs: aminophylline, opioid alkaloids, ammonium chloride, butorphanol, glycopyrronium bromide, hydrocortisone sodium succinate, prednisolone , meglumine, midazolam, pentobarbital, pentobarbital, phenobarbital, phenytoin, hydroxyzine, prochlorperazine, promazine, promethazine, chlorpromazine, trifluoro L-azine, streptomycin, tetracycline, novobiocin sodium, thiopental sodium, heparin sodium.
 2. When taking this medicine while eating or drinking milk, it can reduce the stimulation of the stomach by the medicine.
 The antiemetic effects of this drug can cause difficulties in the diagnosis of certain diseases, such as appendicitis and certain drug-induced reactions.
 4. It is forbidden to engage in high-altitude operations such as driving cars, boats, and operating machinery and equipment during drug use.
5. Overdose performance: Vomiting, dizziness, convulsions, purpura, coma, or even respiratory failure. When the amount of poisoning is reached, severe acaridosis similar to atropine poisoning may occur, accompanied by extrapyramidal symptoms, clozarizine may be administered, and symptomatic treatment such as infusion may be performed. [Usage and dosage]
The recommended dose for intramuscular injection in adults is 50 mg once every 4-6 hours. It is also possible to give 100 mg every 4 hours (no effect if this dose is associated with lethargy). ·Intravenous injection Before use, 1ml (50mg) of this drug must be diluted with 0.9% sodium chloride for injection 10ml, and the administration time is more than 2 minutes. In patients with renal insufficiency who do not have renal failure, evidence of dose reduction is needed. Hepatic insufficiency at doses of acute liver dysfunction should consider reducing the dose.
Children · Intramuscular injection The recommended dose is once 1.25mg/kg, 4 times a day (to 300mg a day). Intravenous Injection It has been reported that before surgery, the drug (50 mg/ml) is diluted to 10 ml with normal saline, and 0.5 mg/kg is injected immediately after induction of anesthesia, which can effectively reduce postoperative nausea and vomiting. In patients with renal insufficiency who do not have renal failure, evidence of dose reduction is needed. Hepatic insufficiency at doses of acute liver dysfunction should consider reducing the dose.
Storage
Protected from light, closed.
Product photo
Our factory
This product is produced in our GMP factory, all our facility has past China GMP, and also officially certificated by more than ten countries'Â MOH. 
Our Service
We can specially develop the products per customers' specification.
We can offer OEM service with customers' private brands and labels.
We can offer excellent after-sale service and professional technical support.
Our Quality control system
In manufacturing, quality is what sustains our life. Starting from the material selection, there are many factors that contribute to the quality control process.  Our manufacturing facility is GMP certified. Quality Control (QC) and monitoring procedures are built into every aspect of our work.
Our Quality Control System is outlined below:
Our Registration Team and technical support
We have experienced team to provide qualified and professional registration documents for most of countries. Up to present, we have registered more than hundred products and exported to more than 20 countries. Especially including those in Asia, Africa, South America, Middle east area and East Europe.
We can provide regulatory documents
Dimenhydrinate Injection with GMP Certificate
Model NO.: 50mg5ml
Standard: USP
OEM: Available
Shelf Life: 3 Years
Trademark: Sundent
Transport Package: 50AMPS/Box, 24boxes/Carton
Specification: 50mg5ml
Origin: China
HS Code: 3004909099
Model NO.: 50mg5ml
Standard: USP
OEM: Available
Shelf Life: 3 Years
Trademark: Sundent
Transport Package: 50AMPS/Box, 24boxes/Carton
Specification: 50mg5ml
Origin: China
HS Code: 3004909099
Description